Which One of the Following Is Not a Strong Electrolyte
Which one of the following is a strong acid. We review their content and use your feedback to keep the quality high.

Solved Which Of The Following Is Not A Strong Electrolyte Chegg Com
OH is a weak electrolyte.
. Weak electrolytes include weak acids weak bases and a variety of other compounds. Can someone explain to me why MgS is not a strong electrolyte. H 2 O - water weakly dissociates in itself.
As well as strong electrolytes there are substances which dissociate in solution but do so only partly. Weak acids and weak bases are weak electrolytes. Which term is not correctly matched with its description.
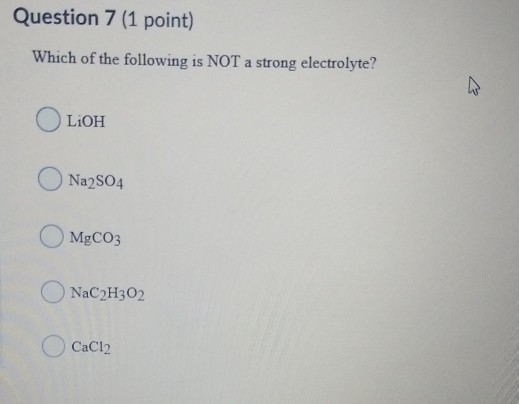
OH is not a strong electrolyte because it doesnt dissociates completely. Which of the following is not a strong electrolyte. Which of the following is NOT a strong electrolyte.
Solution for Which one of the following is NOT a strong electrolyte. Which of the following is not a strong electrolyte A NaCl B KNO3 C NH4OH D FeSO4. Electrolytes come from our food and fluids.
Positively charged ions are called cations. 100 8 ratings Transcribed image text. Posted one year ago.
Strong electrolytes dissociate ___ into ions. Electrolysis is the process of passing an electric. A strong electrolytes b Weak electrolytes c Non-electrolytes.
Want to see the full answer. So its a non electrolyte even though its soluble in water. LIFSHITZ in General Physics 1967 90.
HCl hydrochloric acid H 2 SO 4 sulfuric acid NaOH sodium hydroxide and KOH potassium hydroxide are all strong electrolytes. Strong electrolyte strong acid strong base or soluble salt. NaOH KOH LiOH Ba OH 2 and Ca OH 2.
100 15 ratings Solution MgCO3 is a weak electrolyte A strong electrolyte can. Weak base accepts H from an acid. HCl hydrochloric acid H 2 SO 4 NaOH and KOH are all strong electrolytes.
Give the reason why we need to know types of the costs based on readings. Check Answer and Solution for above question from Tardigrade. NH 3 ammonia C 5 H 5 N pyridine and several more all containing N.
Strong soluble base soluble metal hydroxide. So that is a weak electrolyte and um. Carbonic acid phosphoric acid and acetic acid where on the other hand examples of strong electrolytes are hydrochloric acid sulphuric acid potassium hydroxide also known as potash alum etc.
Is going to be its a weak acid and only partially ionized but it does form ions. When it is dissolved in water all hydrogen present in the formic acid will not release into the solution. Most compounds that contain nitrogen are weak electrolytes.
It does not form any ions. A weak electrolyte is an electrolyte that does not completely dissociate in an aqueous solution. Simply an electrolyte is a substance that can conduct an electric current when melted or dissolved in water.
Weak acid partially ionized. Some examples of weak electrolytes are. Weak electrolytes produce ions but predominantly as molecules that are ____.
Because here only little amount of the dissolved solute is occurring in the form of ions. A LIOH B HNO3 C KBr D NHCI Ε ΗΝΟ. HF - hydrofluoric acid.
NH 3 - ammonia. NH 4. These electrolytes can have an imbalance leading to either high or low levels.
Salts much have high solubility in the solvent to act as strong electrolytes. In solutions of these substances there are not only ions but also neutral moleculesSuch substances are called weak electrolytesThe majority of acids and bases and some salts such as HgCl 2. This is methanol its an alcohol.
Which one of the following is NOT a strong electrolyte. An electrolyte is a substance that dissociates in water into charged particles called ions. Correct option is C Here NH 4.
Check out a sample QA here. Negatively charged ions are called anions. Salt cation of an acid anion of a base.
CH 3 CO 2 H - acetic acid. View the full answer. Sodium potassium and chloride are the significant electrolytes along with magnesium calcium phosphate and bicarbonates.
Classify the following substance under three headings. Weak electrolytes only partially break into ions in water. High or low levels of electrolytes disrupt normal bodily functions and can lead to even life.
NaCl KBr MgCl 2 and many many more. Strong acids strong bases and ionic salts that are not weak acids or bases are strong electrolytes. Among the given options formic acid is not an example of strong electrolyte and it is a weak electrolyte and it is a weak carboxylic acid.
Which one of the following is NOT a strong electrolyte. Acetic acid ammonium chloride ammonium hydroxide carbon tetrachloride dilute hydrochloric acid sodium acetate dilute sulphuric acid. 2list and Explain types of companies and types of Inventories based on readings.
HF HC 2 H 3 O 2 acetic acid H 2 CO 3 carbonic acid H 3 PO 4 phosphoric acid and many more. Give one example each of a strong electrolyte and a weak electrolyte. A LIOH B HNO C KBr D NH CI E HNO 2.
We review their content and use your feedback to keep the quality high. Now for the strong ones we have to show what ions will form. 1 Chemical Foundations 2 Atoms Molecules And Ions 3 Stoichiometry 4 Types Of Chemical Reactions And Solution Stoichiometry 5 Gases 6 Thermochemistry 7 Atomic Structure And Periodicity 8 Bonding.
Who are the experts. Advertisement Remove all ads. The substance CH3CH22NH is considered to be A.
How is inventory different from. A strong electrolyte is a solute or solution that is an electrolyte that completely dissociates in a solution. Experts are tested by Chegg as specialists in their subject area.
A LIOH B HNO3 C KBr D NHẠCI E HNO2 Expert Solution.
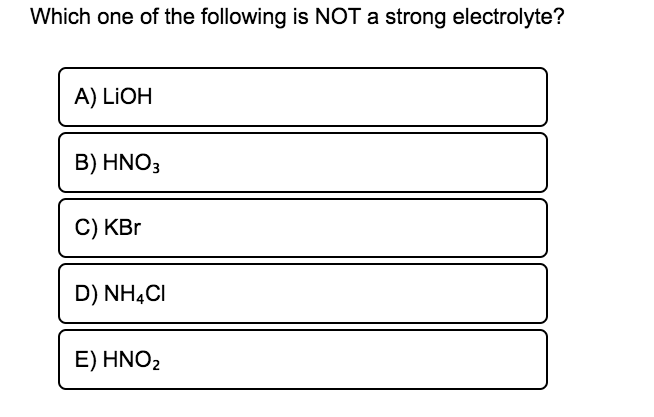
Answered Which One Of The Following Is Not A Bartleby
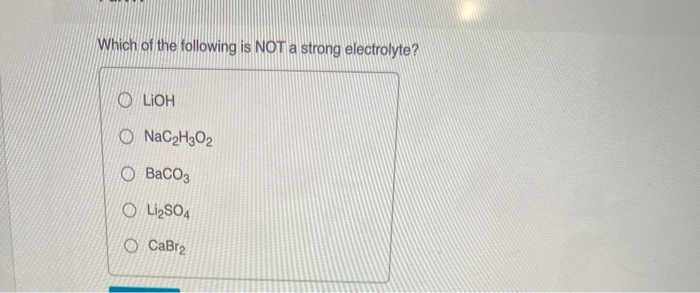
Solved Which Of The Following Is Not A Strong Electrolyte Chegg Com

Solved Question 7 1 Point Which Of The Following Is Not A Chegg Com
No comments for "Which One of the Following Is Not a Strong Electrolyte"
Post a Comment